CH610B Sophomore Organic II
|
Dr. Brian
Pagenkopf |
|
|
|
An Introduction To Molecular Orbitals.
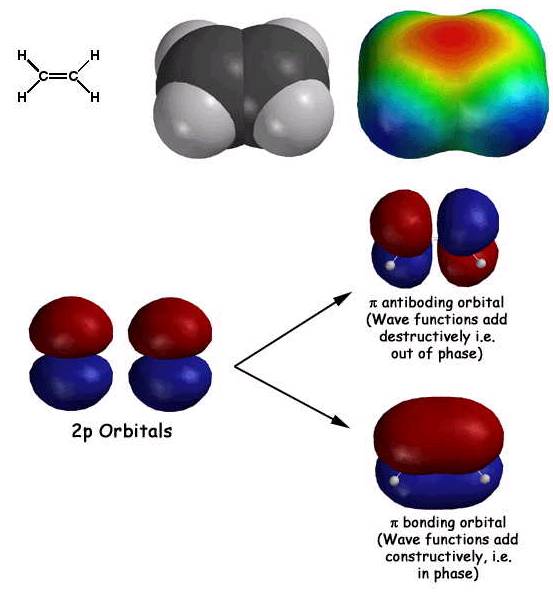
Some basics of molecular orbital theory as it relates to pi systems. First electrons behave as waves, so they are described by wave functions (also known as orbitals). Adjacent atomic 2p orbitals add according to wave mechanics, namely constructively (in phase) and destructively (out of phase). The in phase addition creates the lower energy bonding molecular orbital (the "hotdog bun"), while the out of phase addition results in the higher energy antiboding orbital. The two electrons end up in the bonding orbital, resulting in what we refer to as a pi bond. Note how these pi bonding electrons in the pi bonding orbital are held out and away from the nuclei, so they provide some partial negative charge (red color in top right picture above) to the electrostatic potential surface. Since electrophiles can interact with these electrons, alkenes react as weak nucleophiles.
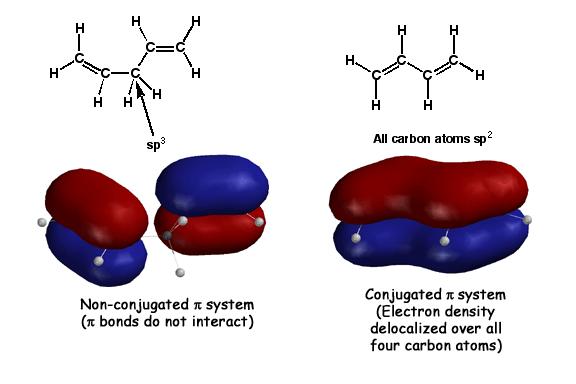
Conjugation occurs when adjacent pi bonds overlap, creating a large "pi way" for the electrons to delocalize or spread around. The molecule on the right above, butadiene, has adjacent pi bonds that overlap to form the large molecular orbital shown. Such overlap of pi bonds is referred to as "conjugation". This is stabilizing compared to isolated pi bonds because pi electrons prefer to be delocalized over as large an area as possible. Since these delocalized pi electrons are in a more stable situation, they are less reactive with electrophiles. The molecule on the left, 1,3 pentadiene, has an sp3 atom between the pi bonds. The pi bonds cannot overlap in this case, so there is no delocalization, no stabilization, and the pi electrons are as reactive as any isolated alkene.

Benzene is the special case in which all the 2p orbitals are contained within a ring. The resulting bagel-shaped pi orbital is delocalized over the entire ring. When certain criteria are met (the Hückel conditions) this is extraordinarily stabilizing. This type of stabilization is referred to as "aromaticity", and benzene is said to be aromatic. As a result, the pi electrons of benzene are relatively unreactive compared to an non-conjugated alkene. Nevertheless, the pi electrons are still weakly nucleophilic as indicated by the red color on the electrostatic potential surface (the red in the lower right structure above). Benzene will react with electrophiles, but only very ‘hot’ electrophiles!
Taken
almost entirely from http://www.cm.utexas.edu/academic/courses/Spring2001/CH610B/Iverson/index.html